Abstract
Background: Addition of the FLT3 inhibitor midostaurin to intensive chemotherapy is the standard of care for newly diagnosed FLT3-mutated AML. Gilteritinib is a 2nd generation FLT-3 inhibitor approved for patients with relapsed/refractory (R/R) FLT3-mutated AML. We studied the combination of gilteritinib with a higher-dose araC-based regimen of Cladribine, Idarubicin, Cytarabine (CLIA) in patients with FLT3-mutated AML.
Methods: Patients aged 18-65 years, fit for intensive chemotherapy, with newly diagnosed FLT3-mutated AML were enrolled. Induction was: Cladribine 5 mg/m 2 IV on days 1-5, Cytarabine 1.5-2.0 g/m 2 IV on days 1-5, Idarubicin 10 mg/m 2 IV on days 1-3 and gilteritinib on days 1-14. Consolidation consisted of up to 5 more cycles of CLIA: cladribine 5 mg/m 2 IV on days 1-3, Cytarabine 750 mg/m 2 IV on days 1-3, and idarubicin 8 mg/m 2 IV on days 1-2 with gilteritinib 120 mg continuously during cycle 2 onward. An additional cohort studied the addition of venetoclax CLIA + gilteritinib. In this cohort, venetoclax was added days 1-7 of each cycle at a target dose of 400mg daily, with dose adjustments for concomitant CYP3A inhibitors.
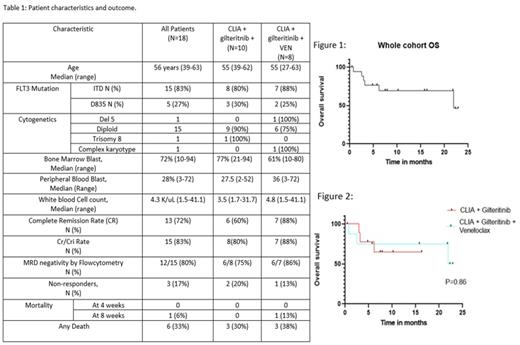
Results: Eighteen patients were enrolled, with a median age of 56 years (range, 39-63). 15 pts (83%) had FLT3-ITD, 5 pts (27%) had FLT3 D835, and 2 pts (11%) had both. The median allelic ratio of FLT3-ITD was 0.36 (0.06-1.45) and of FLT3 D835 was 0.15 (.05-.084 Patient characteristics and outcomes are outlined in TABLE 1. Fifteen patients (83%) had diploid cytogenetics, one patient with -5, one with trisomy 8, one with Inv (16) and one patient with complex karyotype. The most commonly co-occurring mutations were: NPM1 (67%), TET2 (33%), ASXL1 (33%), and DNMT3A (33%), KDM6A (27%).
Ten patients (56%) received CLIA + gilteritinib and 8 patients (44%) received CLIA + gilteritinib + venetoclax. 13 out of 18 pts (72%) achieved complete remission (CR). The rate of CR / CRi was 83%, including 8/10 pts (80%) in the CLIA + gilteritinib cohort and 7/8 pts (88%) in the CLIA + gilteritnib + venetoclax cohort. The median number of cycles to response was one (x-y). Patients received a median of 2 cycles (1-3) of therapy on protocol.
The 4- and 8-week mortality rate was 6% (one patient in the venetoclax cohort, no early mortality with CLIA + gilteritinib). The most common adverse effects were: Neutropenic fever (12/18, 67%), Grade 2-3 ALT elevation in (3/18, 17%), grade 3 Headache (1/18, 6%) and grade 3 diarrhea (1/18, 6%).
The median time to neutrophil ≥ 0.5 K/uL was 33 days (26-40) and 38 days (30-40) for the CLIA-gilteritinib and CLIA-gilteritinib-venetoclax cohorts. The median time to neutrophil ≥ 1 K/uL was 36.5 days (26-48) and 40 days (31-45) for the CLIA-gilteritinib and CLIA-gilteritinib-venetoclax cohorts, respectively. The median time to platelet ≥ 100 K/uL was 34.5 days (24-48) and 40 days (34-45) for 2 cohorts, respectively.
Twelve of fifteen responding patients (80%) had undetectable minimal residual disease (MRD) tested by multiparameter flow cytometry by the time of response: 7/8 (88%) of the CLIA + Gilteritinib cohort and 5/7 patients (71%) of the CLIA + gilteritnib + venetoclax. Additionally, 13/15 patients (87%) had no detectable FLT3 mutation by PCR at time of response (7/8 patients (88%) of the CLIA + Gilteritinib cohort and 6/7 patients (86%) of the CLIA + gilteritnib + venetoclax cohort).
The median overall survival for the entire cohort was 21.95 months (not reached for the CLIA + gilteritnib cohort and 22.4 months for the CLIA + gilteritnib+ venetoclax cohort) (Figures 1 and 2). Eleven of the 15 responding patients (73%) underwent to allogenic stem cell transplantation in CR1 (7 and 4 patients in the CLIA + gilteritnib and the CLIA + Gilteritinib + venetoclax cohort respectively).
Conclusion: The combination of the FLT3 inhibitor gilteritinib to CLIA produced high rates of complete remission in patients with newly diagnosed FLT3-mutated AML. The addition of venetoclax to the backbone was associated with similar outcomes but prolonged count recovery. The combination resulted in high rates of MRD negativity by flow and molecular testing at the time of response. Further study of the combination of CLIA + gilteritinib in fit patients with newly diagnosed FLT3-mutated AML patients is planned.
Kantarjian: KAHR Medical Ltd: Honoraria; Astra Zeneca: Honoraria; Daiichi-Sankyo: Research Funding; Immunogen: Research Funding; Novartis: Honoraria, Research Funding; NOVA Research: Honoraria; Pfizer: Honoraria, Research Funding; Jazz: Research Funding; Ascentage: Research Funding; Ipsen Pharmaceuticals: Honoraria; AbbVie: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Precision Biosciences: Honoraria; Aptitude Health: Honoraria; Astellas Health: Honoraria; BMS: Research Funding; Taiho Pharmaceutical Canada: Honoraria. Alvarado: Daiichi-Sankyo: Research Funding; MEI Pharma: Research Funding; Sun Pharma: Consultancy, Research Funding; CytomX Therapeutics: Consultancy; FibroGen: Research Funding; Jazz Pharmaceuticals: Research Funding; BerGenBio: Research Funding; Astex Pharmaceuticals: Research Funding. Yilmaz: Daiichi-Sankyo: Research Funding; Pfizer: Research Funding. Pemmaraju: LFB Biotechnologies: Consultancy; ASH Communications Committee: Membership on an entity's Board of Directors or advisory committees; MustangBio: Consultancy, Other; Abbvie Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding; Plexxicon: Other, Research Funding; DAVA Oncology: Consultancy; Celgene Corporation: Consultancy; Roche Diagnostics: Consultancy; Affymetrix: Consultancy, Research Funding; Novartis Pharmaceuticals: Consultancy, Other: Research Support, Research Funding; Stemline Therapeutics, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding; Protagonist Therapeutics, Inc.: Consultancy; Sager Strong Foundation: Other; Dan's House of Hope: Membership on an entity's Board of Directors or advisory committees; HemOnc Times/Oncology Times: Membership on an entity's Board of Directors or advisory committees; Clearview Healthcare Partners: Consultancy; CareDx, Inc.: Consultancy; Samus: Other, Research Funding; Daiichi Sankyo, Inc.: Other, Research Funding; Springer Science + Business Media: Other; Incyte: Consultancy; Aptitude Health: Consultancy; Cellectis S.A. ADR: Other, Research Funding; ASCO Leukemia Advisory Panel: Membership on an entity's Board of Directors or advisory committees; Blueprint Medicines: Consultancy; Bristol-Myers Squibb Co.: Consultancy; ImmunoGen, Inc: Consultancy; Pacylex Pharmaceuticals: Consultancy. Daver: ImmunoGen: Consultancy, Research Funding; Hanmi: Research Funding; Genentech: Consultancy, Research Funding; FATE Therapeutics: Research Funding; Abbvie: Consultancy, Research Funding; Trillium: Consultancy, Research Funding; Trovagene: Consultancy, Research Funding; Gilead Sciences, Inc.: Consultancy, Research Funding; Astellas: Consultancy, Research Funding; Novartis: Consultancy; Bristol Myers Squibb: Consultancy, Research Funding; Glycomimetics: Research Funding; Novimmune: Research Funding; Pfizer: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; Sevier: Consultancy, Research Funding; Jazz Pharmaceuticals: Consultancy, Other: Data Monitoring Committee member; Dava Oncology (Arog): Consultancy; Celgene: Consultancy; Syndax: Consultancy; Shattuck Labs: Consultancy; Agios: Consultancy; Kite Pharmaceuticals: Consultancy; SOBI: Consultancy; STAR Therapeutics: Consultancy; Karyopharm: Research Funding; Newave: Research Funding. Burger: TG Therapeutics: Other: Travel/Accommodations/Expenses, Research Funding, Speakers Bureau; Novartis: Other: Travel/Accommodations/Expenses, Speakers Bureau; Gilead: Consultancy, Other: Travel/Accommodations/Expenses, Research Funding, Speakers Bureau; AstraZeneca: Consultancy; Beigene: Research Funding, Speakers Bureau; Pharmacyclics LLC: Consultancy, Other: Travel/Accommodations/Expenses, Research Funding, Speakers Bureau; Janssen: Consultancy, Other: Travel/Accommodations/Expenses, Speakers Bureau. Jain: Aprea Therapeutics: Research Funding; Cellectis: Honoraria, Research Funding; Genentech: Honoraria, Research Funding; AstraZeneca: Honoraria, Research Funding; Incyte: Research Funding; Janssen: Honoraria; Beigene: Honoraria; Servier: Honoraria, Research Funding; ADC Therapeutics: Honoraria, Research Funding; Fate Therapeutics: Research Funding; Adaptive Biotechnologies: Honoraria, Research Funding; Bristol Myers Squibb: Honoraria, Research Funding; Pfizer: Research Funding; TG Therapeutics: Honoraria; Precision Biosciences: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Pharmacyclics: Research Funding. DiNardo: Takeda: Honoraria; ImmuneOnc: Honoraria, Research Funding; Notable Labs: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Forma: Honoraria, Research Funding; Bristol Myers Squibb: Honoraria, Research Funding; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; Foghorn: Honoraria, Research Funding; AbbVie: Consultancy, Research Funding; Agios/Servier: Consultancy, Honoraria, Research Funding; Novartis: Honoraria; Celgene, a Bristol Myers Squibb company: Honoraria, Research Funding. Borthakur: Ryvu: Research Funding; Protagonist: Consultancy; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; ArgenX: Membership on an entity's Board of Directors or advisory committees; Astex: Research Funding; University of Texas MD Anderson Cancer Center: Current Employment; GSK: Consultancy. Ravandi: Prelude: Research Funding; AbbVie: Honoraria, Research Funding; Astex: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Jazz: Honoraria, Research Funding; Taiho: Honoraria, Research Funding; Xencor: Honoraria, Research Funding; Agios: Honoraria, Research Funding; Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Syros Pharmaceuticals: Consultancy, Honoraria, Research Funding; AstraZeneca: Honoraria; Novartis: Honoraria. Kadia: Ascentage: Other; Genfleet: Other; Cellonkos: Other; Pfizer: Consultancy, Other; AstraZeneca: Other; Novartis: Consultancy; Sanofi-Aventis: Consultancy; Astellas: Other; Pulmotech: Other; Liberum: Consultancy; Jazz: Consultancy; Genentech: Consultancy, Other: Grant/research support; Dalichi Sankyo: Consultancy; Cure: Speakers Bureau; BMS: Other: Grant/research support; Amgen: Other: Grant/research support; Aglos: Consultancy; AbbVie: Consultancy, Other: Grant/research support.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal